Alongside the production of healthy aquafeed, which is the initial aim of my project and the intended purpose of the high seas infrastructure, the latter is also a wonderful stepping stone to develop other opportunities.
Cosmetics and medicines
Thanks to the biological affinity between our blood and seawater, between our cells and marine ones, cosmetics containing active agents from marine sources provide some of the most sympathetic interactions with the skin. The market for natural cosmetics is constantly growing and already faces shortages in supply uncontaminated ingredients. The understandable tendency for consumers to seek natural ingredients in their cosmetics, will no longer allow the industry to artificially reproduce natural molecules they find interesting. In order to be both accepted and appreciated, ingredients need to be produced naturally in a healthy and sustainable way. Chemically synthesized molecules or even cultures in stainless steel or plastic tanks will not be able to compete with cultures in natural seawater originating from before the Anthropocene.

(Source : Departamento de Oceanografia e Pescas, Universidade dos Açores)
Moreover, many chemicals produced by marine organisms are useful as medicines. Manzanine A, for example, is a pentacyclic alkaloid with various bioactivities, including recently reported anticancer activity. It is produced by a very common sponge in Indonesian waters. Acanthostrongylophora Igens is able to grow well in poor-quality polluted water, but heavy metals and other contaminants could bind to manzanine A, making it more difficult and expensive to extract. Yet another argument to prefer the pristine waters from the AAIW upwelled with the Perpetual Salt Fountain pipes for cultivation of marine species.

(Source Marinespecies.org)
Carbon sink
Another opportunity is to scale up a natural carbon sink. This is based on the fact that bivalve shells are made of 95% calcium carbonate whose carbon originates from the environment. If the Davis Bank installation produces 200 tons mussels on long lines per hectare per year (an achievable production average if the carrying capacity is given), at least 50% of that harvest will be shell, representing 100 tons of calcium carbonate (aragonite and calcite) containing nearly 12% of carbon, that is 12 tons of carbon per hectare, being permanently removed each year. In comparison, a forest on land retains only 4 tons of carbon per hectare per year, and only as long as it is growing.
But there is a scientific controversy about the relative importance and attribution of the different carbon fluxes occurring during shellfish production and the formation of their shells. On one side it is a carbon sink due to evident carbon sequestration by shells (calcium carbonate is a fossil carbon reservoir of biological origin, like coal or petroleum), on the other side it is a carbon source due to the created CO2 during the biomineralisation process, alongside with the animal’s metabolism and breathing which also produces CO2.
At first sight, stoichiometric analyses indicate that the amount of carbon sequestered in the shells is not significantly more important than that released during calcification process and respiration. This is why shellfish cultivation, unlike afforestation, has not been taken into account as a carbon offset scheme by the international carbon trading system first implemented by the Kyoto Protocol and then taken over by the Paris Agreement with voluntary goals. In my opinion, however, these isolated carbon fluxes have to be understood in correlation with other related natural cycles.
The chemistry involved in the process of shell-making, called marine calcification or biomineralisation, relies on a set of ionic (or electrolytic) dissociations and associations in water, which are in equilibria governed by local conditions. CaCO3 and CO2 are produced from calcium and hydrogencarbonate ions in solution as described by the following scheme:
Ca2+ + 2HCO3− ⇌ CaCO3 + CO2 + H2O
(in words: one calcium ion + two hydrogencarbonate ions ⇌ calcium carbonate + carbon dioxide + water).
During the calcification (biomineralisation) reaction, the carbon atom from the first hydrogencarbonate ion is released as a molecule of CO2 and the carbon atom of the second hydrogencarbonate ion is sequestered as calcium carbonate. Both carbon atoms originate from the sea, whose CO2 partial pressure is in equilibrium with that of the atmosphere. This clearly means that half of the involved CO2 for biomineralisation simply stays in the environment and the other half is a real carbon sink.
(Source: CommonseaGood)
The electrolyte dissociations and associations of carbon dioxide in water are described by these schemes:
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− ⇌ 2H+ + CO32-.
(in words: one molecule of carbon dioxide + one molecule of water ⇌ one molecule of carbonic acid ⇌ one hydrogen ion (= proton) + one hydrogencarbonate ion ⇌ two hydrogen ions + one carbonate ion).
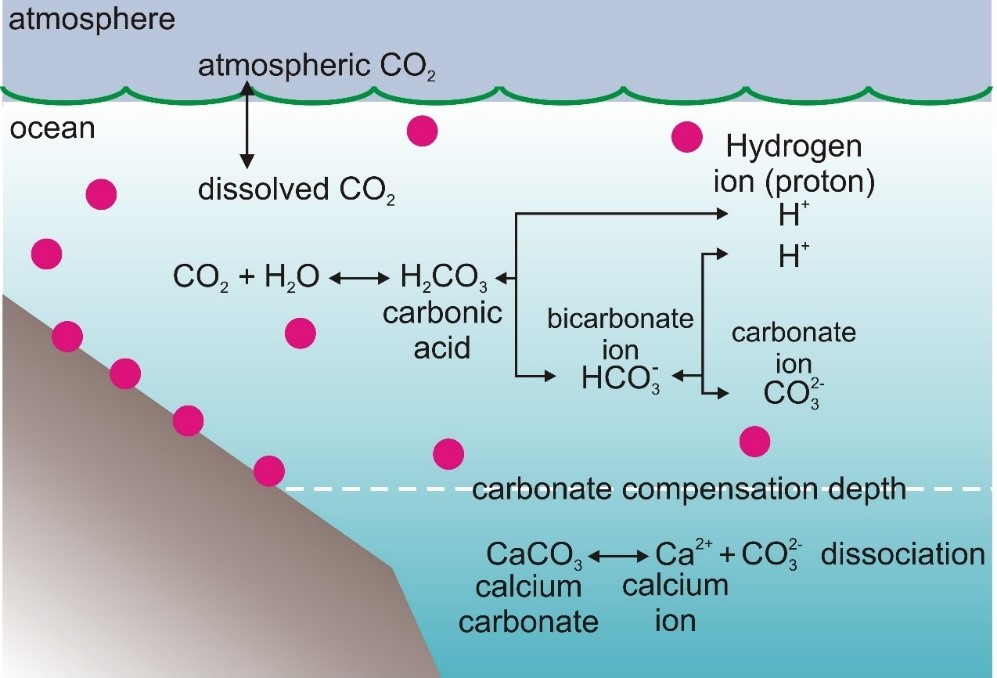 (Source: redrawn figure of Encyclopaedia Universalis)
(Source: redrawn figure of Encyclopaedia Universalis)
Seawater is over-saturated with calcium and its concentration of hydrogencarbonates largely dominates those of carbonates or carbonic acid. In these conditions, the molecule of CO2 released during the biomineralisation process will bind with water, forming carbonic acid which will dissociate rapidly forming protons and hydrogencarbonate ions, the latter being available for marine calcifiers to form calcium carbonate again. To a much lesser extent, carbonic acid can dissociate to form a carbonate ion and two protons.
When atmospheric carbon dioxide binds with water and forms carbonic acid that dissociates mainly in hydrogen carbonate ions and protons (hydrogen ions, H+), it will clearly cause acidification of seawater which is accused to disturb the formation of calcium carbonate by marine calcifiers. Adverse effects of present day ocean acidification (from pH 8.2 to pH 8.1 since the industrial area), interwoven with elevated temperatures are clearly seen to impact the viability of zooxanthellae, the symbiotic algae of coral which lead to the bleaching of the latter. However mussels, cultured under stronger acidific conditions, react by producing more amorphous and less crystallized calcium carbonate.
The present levels of elevation of marine CO2 concentration and consequential increase in proton concentration and acidity are more likely to encourage calcification than discourage it. The calcification process is thought to have originated when large amounts of excess calcium occurred in the seawater at the Precambrian-Cambrian boundary, about 550 million years ago. The organisms of the time had already evolved sophisticated mechanisms for maintenance of cellular calcium homeostasis. It is theorised that the environmental calcium excess produced conditions favoring natural selection for biomineralisation in protists and invertebrates. Now the same primitive calcification process appears to be effective at sequestering anthropogenic carbon in excess.
One would object that the released carbon dioxide from biomineralisation not only forms hydrogencarbonate for the calcifiers benefit, but also protons which cause sea acidification and its correlated loss of potential to absorb atmospheric carbon (acting as a carbon source). Fortunately it is the fundamental nature of biological systems that they carry out their processes inside their own cells, within phospholipid membrane boundaries evolved specifically to separate the life processes from the open water environment. Biological calcification takes place on the surfaces of enzymatic polypeptides, within organelles that have phospholipid membranes, contained in a cell enclosed with another phospholipid bilayer membrane. In other words, biomineralisation takes place inside the animal’s mantle cells without acidifying the open water environment. The protons generated during this process are used to perform various biological activities, such as the synthesis of ATP. That’s life! ![]()
Mussel respiration, in its turn, is due to metabolic activities, fueled by the ingestion of phytoplankton carbon, which belongs to the biological carbon reservoir of the ocean, like all other marine organisms. After a variable residence time there among the prey-predator relationships, only a fraction of this stored carbon reaches the sea bottom sediments, and is then sequestered for a long time – millennia and more. When phytoplankton is ingested by shellfish, the carbon track is exactly the same as for other plankton eaters. It is an act of recycling, not an add-on of carbon in the fluxes. It involves only the animal’s soft body with its metabolism, not its shell formation. As minerals, shells have left the biosphere in the strict sense. That’s why mussel respiration is neutral in the carbon budget of shell making.
Shellfish shells are not made of living cells and are produced outside the animal’s body. Their calcium carbonate is elaborated by the mollusk’s mantle, using calcium and hydrogencarbonate ions from seawater, the carbon of the latter coming initially from the atmosphere. The important thing is what remains after the animal’s death. The shell carbon is effectively and permanently sequestered in a mineral form, indigestible and chemically stable for geological periods of time.
The same pattern of carbon fluxes occurs with forests. In the same way as the marine phytoplankton, terrestrial green plants are photosynthetic primary producers that fix carbon dioxide out of the atmosphere into their carbohydrates. The carbon reservoir of a forest is represented by its biomass of wood, leaves, fruits and roots, but also by all of the animals, large and small, all of the microbes, bacteria, fungi and protozoa that digest the wastes of the forest, and all of the carbon accumulated over the years in the humus of the forest soil.
When a forest is mature, it no longer retains more atmospheric CO2 through photosynthesis than it releases through decaying by legions of bacteria and fungi, except for the part which is sustainably managed for wood. Firewood remains at most two or three years until it is dry enough to be burned. Timber for furniture or buildings may retain its carbon between a few decades to several centuries in the best of cases, until it is decayed or burned, but never longer. Sustainably managed wood is important for a mature forest, its live and its living area. At this stage, the forest represents an important carbon reservoir which has to be treasured, but it isn’t a relevant carbon sink anymore. A very important point about sequestration of carbon by the forest biome is that it is very temporary. The plants and the animals will die all too soon and their subsequent decay releases their carbon back into the atmosphere again, together with the one of the extracted firewood or timber. Forests, even when they are in active growth, can only be temporary carbon sinks.
The problem of afforestation and reforestation is also to find enough free land and, above all, to secure the growth of new trees when exiting ones are already overstressed and dying in alarming proportions due to climate change and related pest attacks. The global increase of forest fires does nor improve the situation. With limited upscaling perspectives, uncertain results and, in any case, time limited efficiency, tree planting is a hazardous candidate for the international carbon trading system, despite the fact that it is believed so widely to be a respected opportunity for industries who need to improve their carbon footprint. We cannot count on tree planting alone to mitigate climate change.
There is a better alternative. If expansion of shellfish cultivation were to be accepted as a carbon sink within the frame of the carbon trading system, it would be much easier to implement on a large scale, more sustainable and, most importantly, offer permanent removal of carbon from the atmosphere. It is easier to implement on a large scale because cultivating shellfish does not challenge the use of scarce agricultural land which is better to devote to farming for food. It is more sustainable because shellfish cultivation can be easily combined with reef conservation projects and restauration of shellfish fisheries like clam gardens which would tackle the problem of undernourishment around the globe. The permanency (over geological periods of time) of the removal of atmospheric carbon dioxide by biomineralisation of shells has already been discussed above.
Polluting states, cities and industries wishing to improve their carbon footprint, could thus make their contribution to climate mitigation while, at the same time giving a serious boost to conservation of coastal areas and the fight against malnutrition. Instead of paying people to plant trees that still need to be maintained or even watered to grow properly and fulfil their duty of only temporary carbon sequestration, these contributions could fund just once each time the research, equipment and teaching required to spread enhanced shellfish cultivation around the globe. The needed workers will then be able to pay themselves with the produced food and secure their environment permanently, locally as well as globally.
This was a reflection arising from the shellfish properties of carbon sequestration and food production in coastal areas. How my High Seas project on Davis Bank can contribute here is that apart from the planned mussel production for aquafeed (which is centred on harvested mussel meat), a carbon sequestration programme (focused on the mussel shell) could be deployed easily, on a very massive scale, from this location towards the high seas. Given the large biomass of mussel larvae (each female spawns a million of eggs), combined with the fact that in oligotrophic waters mussels can build quite normal shell without growing much meat (biomineralisation is much less energy consuming than living tissues development), it would be feasible to produce quantities of small biodegradable floating devices, to let them collect mussel larvae from the spawning mussels of the Davis Bank facility before being released into the passing Brazil Current (BC) which then carries them south-easterly towards the South Atlantic Gyre. The shells will grow and, after a while, will sink by their own weight. When the animals die, their soft tissues will release their carbon somewhere inside the biological carbon reservoir and the carbon locked in their shells will then be sequestrated permanently in the ocean’s depths. Even when the shells will fall below the Carbonate Compensation Depth (mostly between 3000 and 5000 m, except at higher latitudes) where calcium carbonate solubilises again, the calcium and hydrogencarbonate ions will be carried by the global thermohaline circulation (1000 years turnover) and are likely to be swept in lesser depths to recrystallise (for an other millenium).
You can read more about it with Dr David Moore’s publications “Saving the Planet with Appropriate Biotechnology”: 1. Diagnosing the Problems ; 2. Cultivate Shellfish to Remediate the Atmosphere ; 3. The high seas solution (same subject as this website but more scientific); 4. Coccolithophore cultivation and deployment ; 5. An action plan .
Considering all this arguments, it seems that shellfish farming is the only industry able to scale up massively against climate change, while improving global food supply at the same time. The most harmful effect of climate change being the undermining of the ecological basis of food production, my proposal to include shellfish cultivation in the carbon trading system, may hit two targets with one bullet and would globally support at least five of the United Nations Sustainable Development Goals.





Fishing with dolphins
Another perspective flirts nearly with full happiness. The oasis of life that could be created on Davis Bank will also result in numerous interactions with wild animals and inspire other challenges. Sea bream, for example, will come and crunch mussels. Dolphins, seals or other marine mammals will also be attracted and could provide the final delights. Indeed, these particular species can be educated, trained or encouraged to assist capture fishermen by selecting wild fish shoals in the surroundings using their echolocation and driving them into the fishermen’s nets.
There are several places on earth where dolphins collaborate in hunting fish together with mankind, in Brazil, but also in Mauritania and in Myanmar as well. These cooperative and mutually beneficial bonds have lasted for generations, even since the 15th century in Mauritania, and are based on trusting relationships between individuals of the two species who both do well out of it. Combined with the dolphins’ ability to recognize geometric figures and colors (demonstrated on a daily basis in marine parks the world over), their goodwill could certainly allow to herd schools of targeted fish species towards prepared fishing nets around Davis Bank facility.
This could be a solution to avoid today’s fisheries’ bycatch and stop their too large energy spending that makes them economically unsustainable without subsidies.
Take a look at the trailer below about cooperative fishing between dolphins and fishermen in Laguna, Brazil!
(Courtesy: Longtail Distribution Network)
We need to change our old hunter habits and imagine new possibilities to take advantage of our oceans and preserve them at the same time. We can make a start settling the “Far Wet” ![]() on Davis Bank because of its ideal environment, but there are many seamounts awaiting us when the technology has been developed and validated.
on Davis Bank because of its ideal environment, but there are many seamounts awaiting us when the technology has been developed and validated.
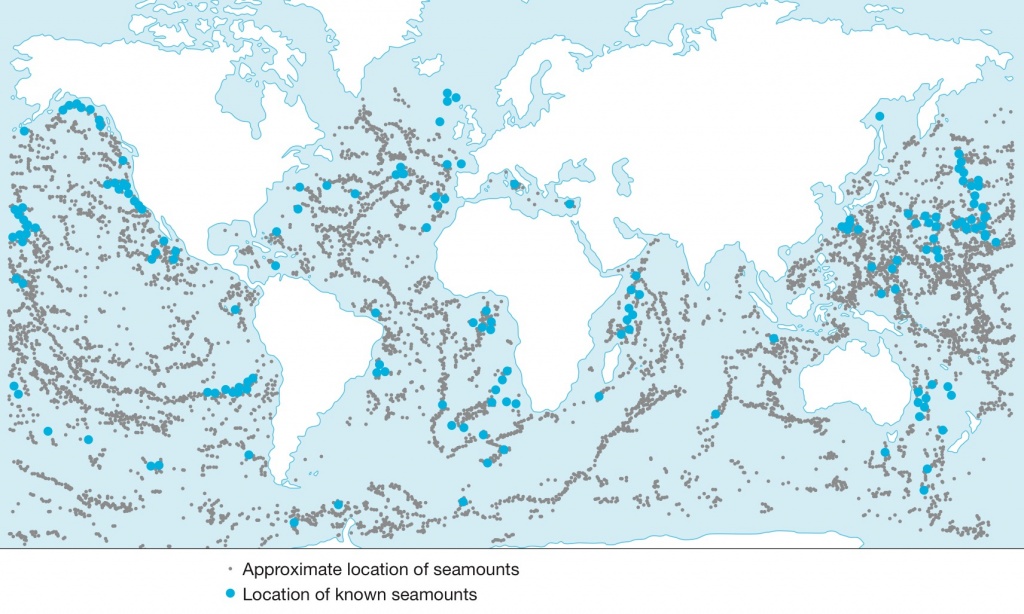
(Source: A planet for Life adapted from Sea Around Us)
Follow me on What you can do